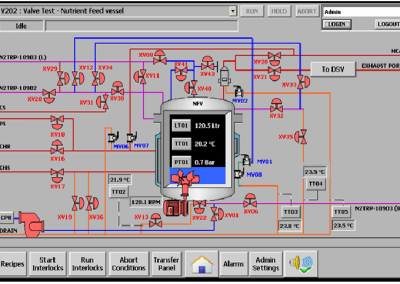
One client in the pharmaceutical industry wanted a scalable and reliable system architecture, hassle free production and process compliance with US-FDA and EU systems.
Outcome:
- Engineering and commissioning of Automation system with totally distributed architecture as per 21 CFR Part 11 guidelines.
- Precise Batch Reports, Acute Raw Material Consumption Tracking, ClP Validation were implemented as per US-FDA Guidelines and GMP.
- Centralized Data Logging of Process Values, Batch Trends, Supervisory control, User Administration was provided.
- Intuitive interface required for almost zero training and provided single click diagnostics.